
Structure 1.4.5-The molar concentration is determined by the amount of solute and the volume of solution.The molecular formula gives the actual number of atoms of each element present in a molecule. Structure 1.4.4-The empirical formula of a compound gives the simplest ratio of atoms of each element present in that compound.Structure 1.4.3-Molar mass M has the units g mol^–1.Structure 1.4.2-Masses of atoms are compared on a scale relative to 12C and are expressed as relative atomic mass Ar and relative formula mass Mr.One mole contains exactly the number of elementary entities given by the Avogadro constant. Structure 1.4.1-The mole (mol) is the SI unit of amount of substance.Structure 1.4-Counting particles by mass: The mole.Structure 1.3.7-Successive ionization energy (IE) data for an element give information about its electron configuration.Structure 1.3.6-In an emission spectrum, the limit of convergence at higher frequency corresponds to ionization.Structure 1.3.5-Each orbital has a defined energy state for a given electron configuration and chemical environment, and can hold two electrons of opposite spin.Structure 1.3.4-A more detailed model of the atom describes the division of the main energy level into s, p, d and f sublevels of successively higher energies.Structure 1.3.3-The main energy level is given an integer number, n, and can hold a maximum of2n^2 electrons.

Structure 1.3.2-The line emission spectrum of hydrogen provides evidence for the existence of electrons in discrete energy levels, which converge at higher energies.Structure 1.3.1-Emission spectra are produced by atoms emitting photons when electrons in excited states return to lower energy levels.Structure 1.2.3-Mass spectra are used to determine the relative atomic masses of elements from their isotopic composition.Structure 1.2.2-Isotopes are atoms of the same element with different numbers of neutrons.Negatively charged electrons occupy the space outside the nucleus Structure 1.2.1-Atoms contain a positively charged, dense nucleus composed of protons and neutrons (nucleons).Structure 1.1.3-The temperature, T, in Kelvin (K) is a measure of average kinetic energy Ek of particles.Structure 1.1.2-The kinetic molecular theory is a model to explain physical properties of matter (solids, liquids and gases) and changes of state.Structure 1.1.1 Elements are the primary constituents of matter, which cannot be chemically broken down into simpler substances.Structure 1.1-Introduction to the particulate nature of matter (SL/HL).Models of the particulate nature of matter IBDP Chemistry SL and HL (2025 Specification).
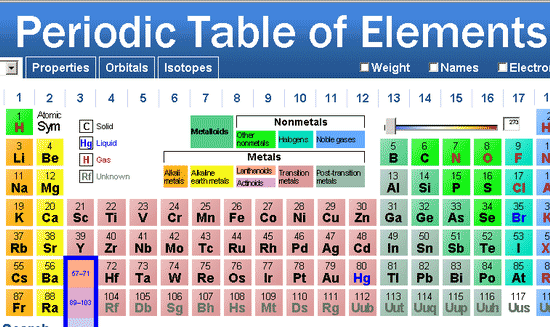
Dynamic periodic table pdf#
Now available: history of the periodic tableĬhoose elements by name, by atomic number, by symbol, by massĬlick here for the history of the periodic table.Ĭlick here to download a PDF version from that periodic table An interactive, printable extended version of the Periodic table of chemical elements of Mendeleev (who invented the periodic table).


 0 kommentar(er)
0 kommentar(er)
